Regulation of stress granules and paraspeckles under stress and in disease states
Stress granules and paraspeckles are prototypical RNP granules localised in the nucleus and cytoplasm, respectively. Recently, we found that despite their spatial separation these two RNP granules are intimately linked. Our aim is to dissect molecular mechanisms behind this crosstalk.
RNP granules in health and disease. Eukaryotic cells contain a whole repertoire of large, microscopically visible RNA-protein complexes termed ribonucleoprotein (RNP) granules. RNP granules represent membraneless organelles – biomolecular condensates lacking a membrane and maintained through a combination of protein-protein, protein-RNA and RNA-RNA interactions. High concentration of molecules within RNP granules makes these structures an ideal platform for the regulation of numerous processes related to mRNA localisation, translation, transport and turnover. Because of their remarkable molecular plasticity, RNP granules are also widely utilised by cells to rapidly adjust and rewire regulatory networks in response to stress, serving as both stress sensors and effectors. RNP granules recruit multiple RNA-binding proteins (RBPs) characterised by the presence of low sequence complexity, intrinsically disordered regions in their structure that drive efficient phase separation and condensate formation. Nuclear RNP granules are often referred to as “nuclear bodies”, here belong paraspeckles, Gems, Cajal bodies, PML bodies and splicing speckles, among others. The most well-known cytoplasmic RNP granules are stress granules, P-bodies and neuronal RNA transport granules.
Because of the high local levels of biomolecules within RNP granules, weakened or enhanced interactions between their components will affect biochemical processes leading to severe cellular dysfunction and disease states. The ability of RBPs to self-associate and oligomerise is crucial for physiological phase separation and RNP granule formation, however if not controlled properly, it results in their pathological aggregation. Many RNP granule components are affected by mutations causing amyotrophic lateral sclerosis-frontotemporal dementia (ALS/FTD) disease spectrum. Furthermore, presence of abnormal inclusions composed of protein components of RNP granules in the affected tissues is typical for ALS/FTD. RNP granules that act as crucial hubs for stress signaling pathways are also often exploited by cancer cells to adapt to the adverse conditions of the tumour microenvironment and enhance their fitness and survival.
Stress granules and paraspeckles. Paraspeckles are nuclear RNP granules formed in the interchromatin space. The longer isoform of the long non-coding RNA NEAT1, NEAT1_2, is a structural, carcass molecule of the paraspeckle. Upon its synthesis, NEAT1_2 is bound and stabilised by a heterodimer of the two RBPs, SFPQ and NONO, and subsequently this complex is joined by FUS protein, giving rise to a mature paraspeckle. Paraspeckle numbers are low in naïve cells but increase many-fold in stressed cells. It is proposed that paraspeckles dynamically recruit some proteins and RNA species thereby regulating their functional, however biological functions of paraspeckles remain poorly understood.
Stress granules (SGs) are large, amorphous cytoplasmic RNP granules whose assembly is usually triggered by phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α). This event leads to halted translation, re-arrangement of mRNPs and their coalescence into micron-scale cytoplasmic condensates. Diverse stresses trigger SG assembly, including oxidative insults, heat shock, viral infection, proteasomal inhibition, ER stress, UV irradiation, mitochondrial poisons. SGs protect mRNA integrity under stress, facilitate selective translation and stress signaling.
Crosstalk between spatially separated RNP granules. Recent advances in the proteomic analysis of RNP granules have suggested that RBPs and multiple other regulatory/signalling proteins are shared components for different types of cytoplasmic granules. However, our recent study has revealed that even spatially separated RNP granules – paraspeckles and SGs localised in the nucleus and cytoplasm, respectively, – are structurally and functionally linked (Figure 1, and An et al., 2019). In particular, SGs have been found to act as regulators of stress-induced paraspeckle assembly in response to diverse stress signals.

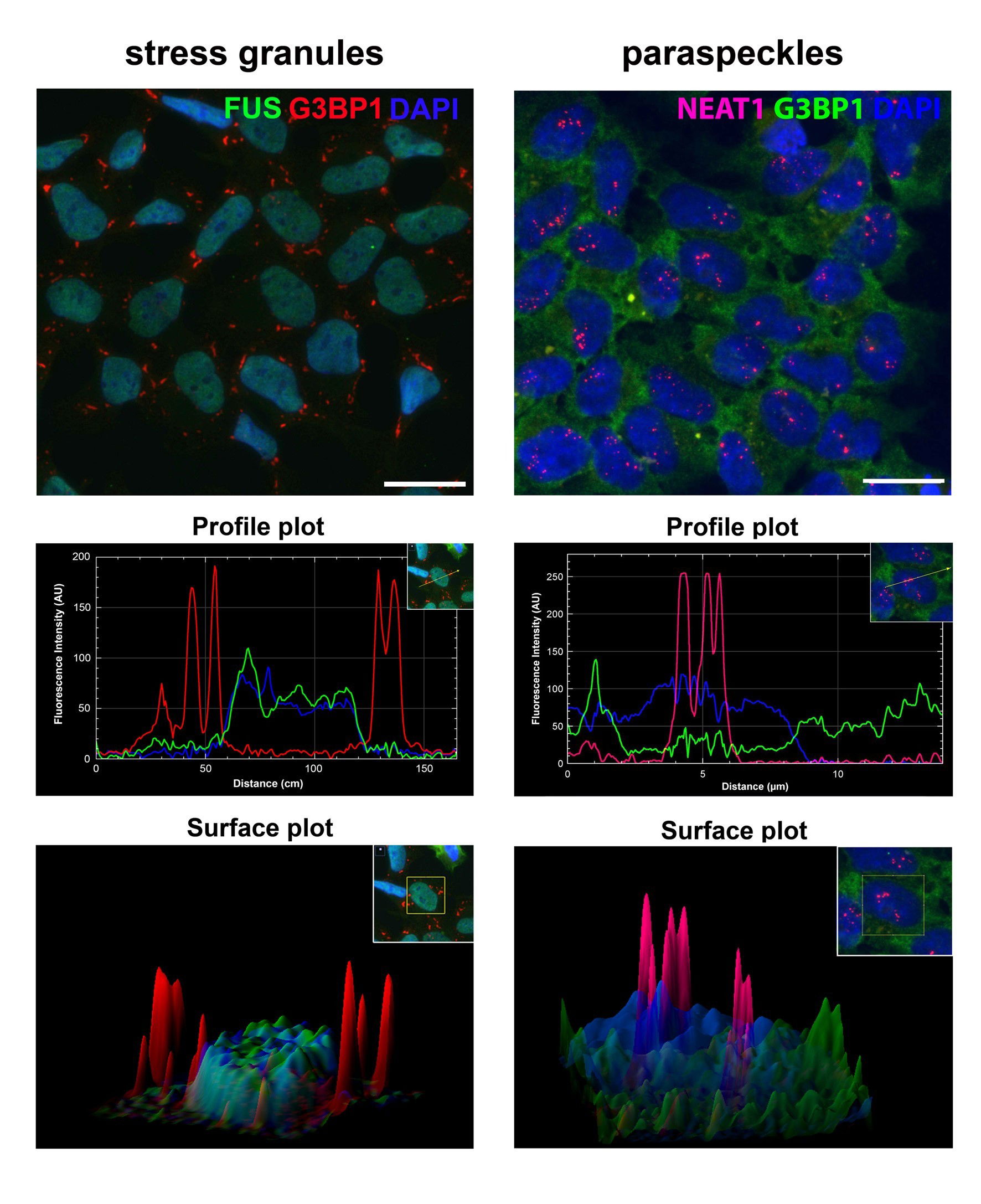
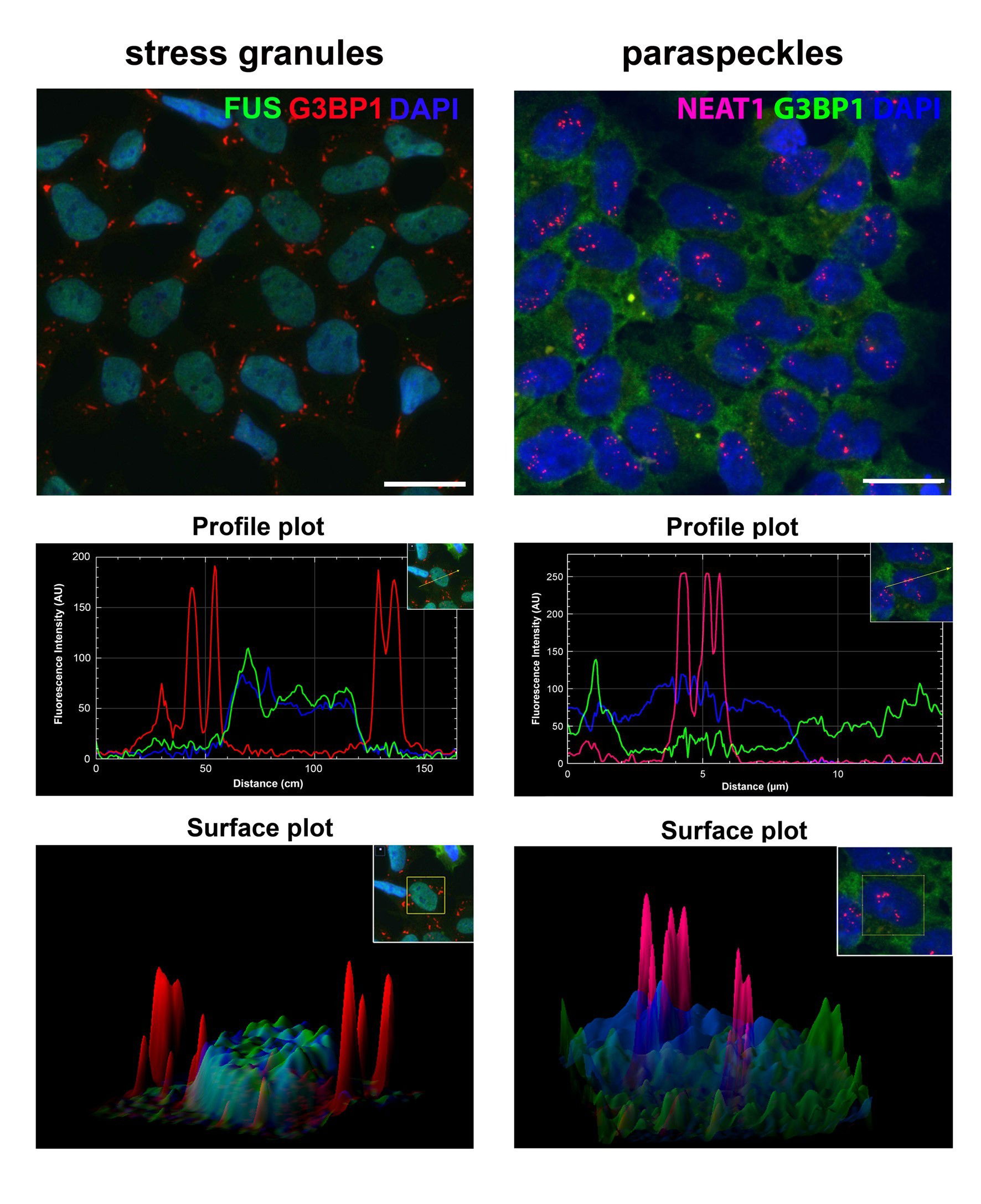
Figure 1. Overlap between paraspeckle and SG proteomes and paraspeckle regulation by SGs. (A) Paraspeckle(PS)-like structures formed by co-expressed SFPQ-GFP and NONO-GFP contain NEAT1. (B) PS-like structures during the lysis of purified nuclei. Partially lysed nucleus (top) and released structures (bottom). (C) Workflow for affinity purification of PS-like structures. (D) STRING protein association for the proteome of PS-like structures. (E) Overlap between PS and SG proteomes. (F) List of PS proteins recruited into SGs. Core PS proteins are in red. (G-I) SGs regulate PS assembly. Cycloheximide (CHX) impedes PS hyper-assembly during stress. Representative images (G), quantification of PS numbers/area (H) and NEAT1_2 levels (I) are shown.*/ # - p<0.05; ****/ #### - p<0.0001.
This finding has triggered a number of important questions: i) what are the molecular mechanism(s) of SG-mediated paraspeckle regulation? ii) what constitutes the paraspeckle regulome and how is it controlled under stress? iii) does this regulation contribute to cell fate decisions? iv) are other RNP granules regulated by SGs in a similar fashion? v) is this regulation stress-specific? vi) can RNP granule crosstalk be targeted in human disease? Answering this set of questions should provide crucial insights into the molecular underpinnings of RNP granule regulation under physiological and pathological conditions. This knowledge can be harnessed to restore the altered RNP granule homeostasis in human disease and guide therapeutic developments for age-related neurological diseases and cancer (Figure 2, An and Shelkovnikova, 2019). We are keen to pursue these research questions in a set of interconnected projects, with input from motivated students (see Join us tab in the Team).
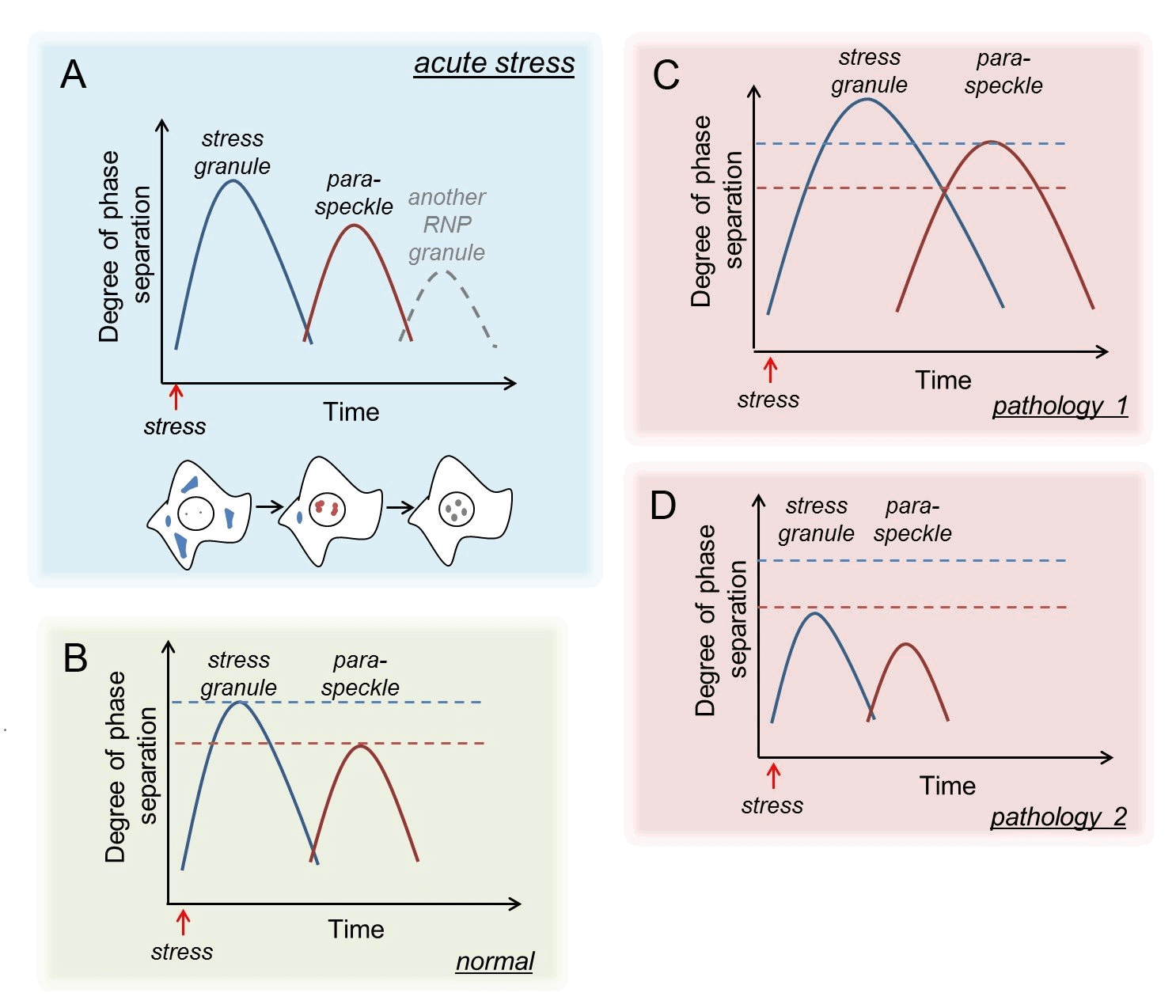
Figure 2.“Waves” of RNP granule formation in response to acute stress (A) and their dysregulation in disease states (B-D).


